Publications
Publications with CSU Pueblo
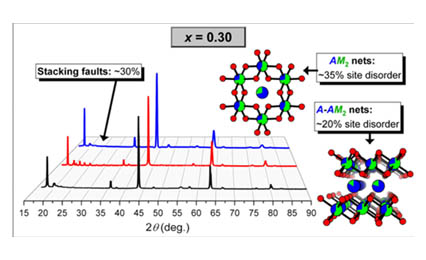
Li2TiO3:Mn4+ has been previously identified as a potential candidate to replace commercial red-emitting Eu2+-activated phosphors. Its luminescent efficiency is limited by the presence of defects and the sensitivity of Mn4+ to redox processes. A series of co-doped Li2TiO3: x Mg2+, 0.01 Mn4+ samples was prepared to gain insight on the effect of electron doping on the structure and photoluminescent properties, through characterization by powder X-ray diffraction, elemental analysis, and optical spectroscopy. As Mg2+ substitutes for Li+, the ordered arrangement of cations within the honeycomb layers in the parent structure (Li2SnO3-type, an ordered derivative of rocksalt) gradually transforms to a more disordered arrangement. The increased cation site disorder leads to less intense and broader emission peaks as well as lower quantum yields.

K. Wallace and J. Cooke, Synthesis of Luminescent Mn2+ and Sb3+ Doped Chloride Double Perovskites: An Adaptable, Inquiry-Based Experiment for Short Laboratory Sessions, ACS Journal of Chemical Education, Journal of Chemical Education Article ASAP. 2025, DOI: 10.1021/acs.jchemed.4c01431.
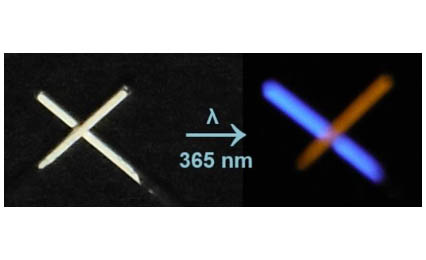
Mn2+ and Sb3+ doped Cs2NaB’Cl6 (B’ = Bi3+ and In3+) halide double perovskites are prepared via a solution-based synthesis method. Certain Sb3+ doped samples exhibit a vivid blue emission, while the Mn2+ doped samples emit a less intense red-orange light under UV illumination. Characterization of these compounds is accomplished by a combination of powder X-ray diffraction (PXRD), scanning electron microscopy (SEM-EDS), and photoluminescence measurements. The synthesized perovskite materials possess a cubic structure, making them ideal specimens for students who are new to solid-state structures. Students utilize computer-based visualization to examine the three-dimensional halide double perovskite structure and determine unit cell dimensions of the synthesized materials through powder X-ray diffraction data. Additionally, students can investigate the photoluminescent properties using a handheld UV lamp and a fluorometer instrument. The experiments offer adaptability to fit various laboratory schedules and cater to students with diverse skill levels. Each part of the experiment is structured to be finished within one to two 3-hour laboratory sessions.
Select Publications – Prior to CSU Pueblo
Abstract
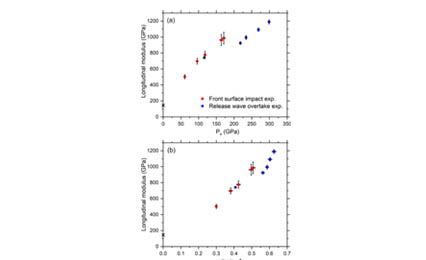
Although [100] lithium flouride (LiF) is the most widely used optical window material in dynamic compression experiments, its high stress (>100GPa) shock compression response, including melting, is not well understood. To address this need, we measured wave profiles in plate impact experiments to determine the Hugoniot states and longitudinal sound speeds in [100] LiF crystals shock compressed to 231 GPa. The measured peak states are fitted well by a linear shock velocity–particle velocity relation, providing an accurate determination of the LiF Hugoniot curve to significantly higher stresses than previous experiments. The longitudinal sound speeds show a near linear increase with density compression to 182 GPa. Between 182 GPa and 195 GPa, the sound speed and the longitudinal modulus decrease abruptly, due to shock-induced melting. The increasing sound speeds and moduli at higher stresses suggest that shock compressed LiF is fully liquid at 195 GPa and above, allowing determination of the Grüneisen parameter for liquid LiF. The melt stress determined here differs from that predicted by current multiphase equations of state for LiF. Our results provide important insight into the high stress solid and liquid states of shock compressed LiF and point to the need for an improved multiphase equation of state at high pressures and high temperatures.
Abstract
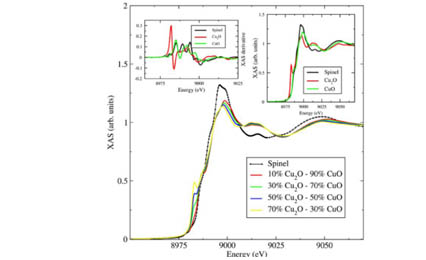
Geometrically frustrated systems populated with large spin-orbit coupled ions are an ideal setting for the exploration of novel exotic states of matter. Here we present an example of iridium on a mixed B-site spinel oxide structure: Cu[Ir1.498(2)Cu0.502(2)]O4. Synchrotron XRD refinements reveal a face-centered-cubic structure with space group 𝐹𝑑-3m and mixed Cu-Ir site disorder within the B2O4 rocksalt substructure. Electrical properties reveal a metallic state within the 50–600-K range with a Kondo effect at 𝑇<50K. X-ray absorption spectroscopy (XAS) measurements show a mixed Cu1+/2+ and Ir3+/4+ charge partitioned picture, which suggests a metallic/band description with reduced on-site Coulomb interactions. Spin-glass-like freezing is seen at 𝑇g=49K, and the hysteresis behavior for 𝑇>𝑇g resembles that of a strongly frustrated magnet. DFT calculations show sizable hybridization between the Cu 3𝑑 and Ir 5𝑑 states with an effective mixed Ir3+/4+ charge partitioned picture, supporting the electronic and XAS results.
Abstract
To gain insight into the thermodynamic response of shock-compressed Ag states corresponding to the face-centered-cubic (fcc) to body-centered-cubic (bcc) transformation and melting observed using in situ x-ray diffraction (XRD) measurements [Phys. Rev. Lett. 124, 235701 (2020)], we present longitudinal sound speed results and their analysis in Ag at peak stresses ranging from 60 to 300 GPa. The measured sound speeds increased linearly with density compression to 171 GPa, showing that the sound speeds (and longitudinal moduli) in the fcc and bcc phases are very similar. Between 171 and 218 GPa, the sound speed dropped significantly, consistent with the melting reported using XRD measurements in shock-compressed Ag. From 218 to 300 GPa, the increasing sound speeds and Hugoniot states provide a determination of the liquid phase Ag response. In particular, determination of the Grüneisen parameter (Γ) showed that the density–Grüneisen parameter product (ρΓ) for liquid Ag is constant, but differs significantly from that for solid Ag at ambient conditions. Thus, the Mie-Grüneisen equation of state can be used to describe the Hugoniot and off-Hugoniot response of liquid Ag.
Abstract
The shock wave response of [100] lithium fluoride (LiF) single crystals at high stresses is of long-standing interest due to their extensive use as optical windows in dynamic compression experiments. The report of melting in shock-compressed LiF single crystals between 134 and 152 GPa—based on a single sound speed datum [Liu et al., J. Appl. Phys. 117, 045901 (2015)]—was surprising because good optical transmission was previously demonstrated in LiF shock compressed to ∼200 GPa [Rigg et al., J. Appl. Phys. 116, 033515 (2014)]. To address these apparent differences, we report on plate impact experiments on [100] LiF single crystals shock compressed to 168 GPa. Wave profiles were measured using laser interferometry to determine Hugoniot states and longitudinal sound speeds in shock-compressed LiF. The measured Hugoniot states are in good agreement with those measured in previous studies. However, the measured sound speeds presented here show no evidence of melting up to 168 GPa. In particular, the abrupt drop reported previously in sound speed at 152 GPa was not observed in the present work. Our results establish a lower bound of 168 GPa for the onset of melting in shock-compressed LiF single crystals.
Abstract
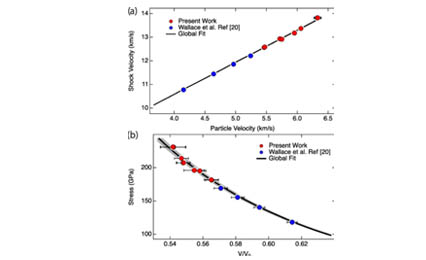
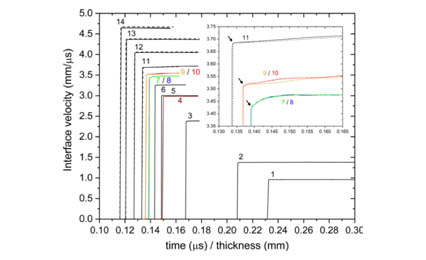
Recent in-situ x-ray diffraction (XRD) measurements on laser-shock compressed Ag foils demonstrated a face-centered-cubic to body-centered-cubic transformation at ∼150 GPa and melting between 172 and 197 GPa [Phys. Rev. Lett. 124, 235701 (2020)]. As a complement to the XRD work, we conducted plate impact experiments to obtain shock velocity and wave profile measurements on thicker Ag samples shock-compressed to peak stresses between 30 and 300 GPa. The shock velocity–particle velocity results were fitted very well by a linear relation over the entire stress range, providing an accurate determination of the Ag Hugoniot (locus of the stress-volume states achieved under shock compression). For peak stresses below 187 GPa and above 210 GPa—corresponding to the solid and liquid phases, respectively—the wave profiles show clean single waves. No wave profile features related to the fcc-bcc transformation at ∼150 GPa were observed, implying minimal volume change for the transformation. For stresses between 187 and 210 GPa, an initial jump was followed by a time-dependent increase in the particle velocity (20–80 ns risetime) to the peak state—corresponding to the solid-liquid mixed phase response. Unlike the solid and liquid response, the mixed-phase response cannot be readily analyzed analytically. Instead, numerical simulations incorporating an accurate multiphase equation of state for Ag—not currently available—are required to analyze the wave profiles measured at 187–210 GPa stresses. The present work shows the potential for using wave profile measurements to examine the melting transition under shock compression.
Abstract
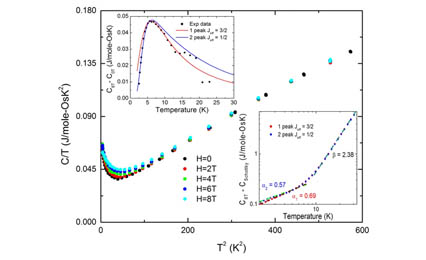
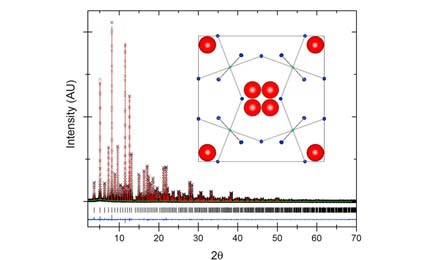
Compounds with honeycomb structures occupied by strong spin orbit coupled (SOC) moments are considered to be candidate Kitaev quantum spin liquids. Here we present the first example of Os on a honeycomb structure, Li2.15(3)Os0.85(3)O3 (C2/c, a = 5.09 Å, b = 8.81 Å, c = 9.83 Å, β = 99.3°). Neutron diffraction shows large site disorder in the honeycomb layer and X-ray absorption spectroscopy indicates a valence state of Os (4.7 ± 0.2), consistent with the nominal concentration. We observe a transport band gap of Δ = 243 ± 23 meV, a large van Vleck susceptibility, and an effective moment of 0.85 μB, much lower than expected from 70% Os(+5). No evidence of long range order is found above 0.10 K but a spin glass-like peak in ac-susceptibility is observed at 0.5 K. The specific heat displays an impurity spin contribution in addition to a power law ∝T(0.63±0.06). Applied density functional theory (DFT) leads to a reduced moment, suggesting incipient itineracy of the valence electrons, and finding evidence that Li over stoichiometry leads to Os(4+)−Os(5+) mixed valence. This local picture is discussed in light of the site disorder and a possible underlying quantum spin liquid state.
Abstract
The “phonon-glass/electron-crystal” approach has been implemented through incorporation of “rattlers” into skutterudite void sites to increase phonon scattering and thus increase the thermoelectric efficiency. Indium filled IrSb3 skutterudites are reported for the first time. Polycrystalline samples of InxIr4Sb12 (0 ≤ x ≤ 0.2) were prepared by solid-state reaction under a gas mixture of 5% H2 and 95% Ar. The solubility limit of InxIr4Sb12 was found to be close to 0.18. Synchrotron X-ray diffraction refinements reveal all InxIr4Sb12 phases crystallized in body-centered cubic structure (space group) with ∼8% antimony site vacancy and with indium partially occupying the 16f site. Unlike known rattler filled skutterudites, under synthetic conditions employed, indium filling in IrSb3 significantly increases the electrical resistivity and decreases the Seebeck coefficient (n-type) while reducing the thermal conductivity by ∼30%. The resultant power factor offsets the decrease in total thermal conductivity giving rise to a substantial decrease in ZT. Principal thermoelectric properties of InxM4Sb12 (M = Co, Rh, Ir) phases are compared. As iridium is a 5d transition metal, zero field cooled (ZFC) magnetization were performed to unravel the effect of spin-orbit interaction on the electronic properties. These results serve to advance the understanding of filled skutterudites, and provide additional insight on the less explored smaller “rattlers” and their influence on key thermoelectric properties.
Abstract
The optical properties of nanocrystalline YPO4:Ln3+ (Ln = Eu, Sm, Tb) prepared via co-precipitation are compared to larger crystallites of YPO4:Ln3+ prepared via traditional solid state reaction. In larger crystals (∼330 nm) a distinct peak is observed at 150 nm in the excitation spectra, the intensity of which decreases markedly in smaller crystals (∼20 nm). Using excitation and reflectance spectroscopy, host–to–activator energy transfer efficiencies were calculated for Y1-xPO4:Lnx3+ (0.01 ⩽ x ⩽ 0.10). From the transfer efficiency data, we estimate that trapping by Eu3+ and Sm3+ is at least five times more efficient than trapping by Tb3+ for excitation at the band edge. The fraction of energy lost to the surface or grain boundaries for excitation at 150 nm and 138 nm is also estimated. We propose that in the samples prepared via co-precipitation, an amorphous phase forms at grain boundaries that is responsible for the loss of efficiency under 150 nm excitation.
Abstract
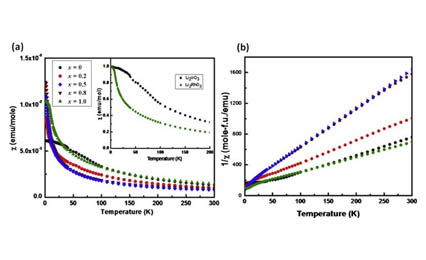
The solid solution series Li2Ir1-xRhxO3 is synthesized for several values of x between 0 and 1. The compounds possess a monoclinic layered structure (space group C2/m) throughout the solid solution range with the lattice constants following Vegard's relationship. Magnetization and resistivity data below room temperature are presented. The effective magnetic moment (μeff) is reduced below the value obtained by interpolating between the end-members, presumably due to nearest neighbor charge exchange leading to non-magnetic Ir5+/Rh3+ pairs. Surprisingly, the degree of reduction of μeff cannot be explained by a random mixture of Ir and Rh and, in particular, is strongly asymmetric around x = 0.5. This anomalous moment reduction possibly results from the difference in on-site Coulomb repulsion between Ir and Rh ions.
Abstract
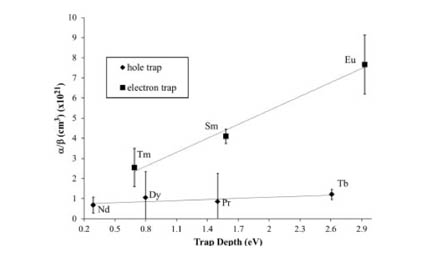
Host-to-activator energy transfer efficiencies in rare-earth doped YBO3 under vacuum ultraviolet excitation have been estimated using excitation and reflectance spectroscopy. From these data, relative electron–hole pair (e–h) trapping rate constants have been calculated for seven different lanthanide dopants. In addition, the energies of activator electronic states relative to YBO3 band states were estimated using published empirical methods. From this, we find a correlation between e–h trapping efficiency and the energy of activator electronic states relative to YBO3 conduction and valence band energies.
Abstract
Absorption of Vacuum Ultraviolet (VUV) radiation creates excited electron - hole (e - h) pairs in phosphors that can either be trapped by bulk defect states, surface defects, Ln(3+) activators, or recombine by host emission. The fraction of e - h captured by Ln(3+) is defined as the transfer efficiency, eta t. Host - to - activator energy transfer efficiencies were determined for micro- and nano-crystalline Y1-xPO4:Eu-x(3+) (0.0025 <= x <= 0.10) using excitation and reflectance spectroscopy. For YBO3:Eu3+ nano - particles, more e - h pairs are lost to the surface relative to the corresponding micron particles (1). Our work on YPO4: Eu3+ nano particles reveals a similar result. However, for both compounds, no conclusion could be made about the effect of particle size on the bulk transport of e - h pairs.
Wallace, M.; Diaz, A.L.; 19.2: Distinguished Paper: Characterization of electron-Hole pair migration and trapping in rare earth doped YBO3 under vacuum ultraviolet excitation; SID Digest of Technical Papers 44, 1, 210-213 (2013).
Wallace, M.K.; Diaz, A.L.; Quantitative assessment of host-to-activator energy transfer efficiency of multiple d-orbital trap states for microcrystalline YBO3:Tb3+, SID Digest of Technical Papers 44, 1, 1217-1220 (2013).


